A few days ago, the temporary halt to enrollment for the DON clinical trial was lifted. Three weeks ago, you read (Halt!) that our group had encountered problems due to the wide range of normal laboratory values in healthy people. We compared the laboratory results before and after participants received DON. If the lab result changed by more than 50%, this was the highest grade of adverse event. Even though lab test results remained in the normal range, we still had to halt, because our Protocol said we should.
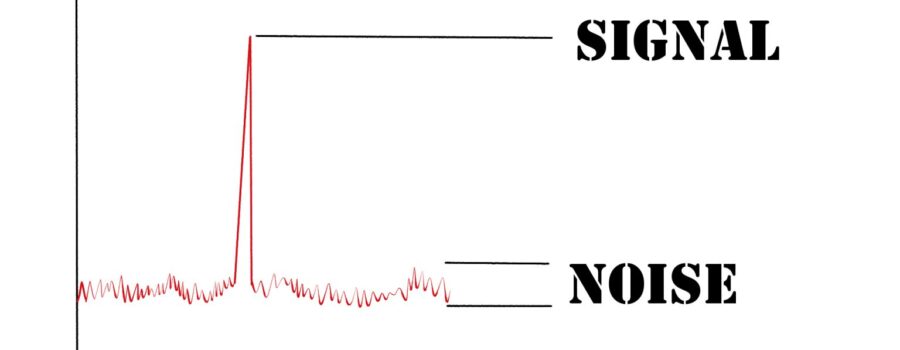
We sent a report to the Data and Safety Monitoring Board (DSMB), an independent group whose mission is to be a disinterested arbitrator of problems when they occur. Their goal, like ours, is to get the clinical trial completed as safely as possible. The DSMB reviewed what occurred and our team’s potential solutions to prevent the problem from recurring. They agreed to change our Protocol to better balance the “signal” (adverse events that are important) to “noise” (laboratory changes that were not meaningful) ratio. Prior to making these changes, we were detecting more noise than signal. Now it is more balanced.
We err on the side of blaming the drug for everything: headaches, backaches, hangnails, car accidents. Well, almost everything.
Conducting a safety clinical trial is nerve wracking. I am continually concerned that participants may have side effects. So far, all the adverse events have been very mild. We err on the side of blaming the drug for everything: headaches, backaches, hangnails, car accidents. Well, almost everything. I suppose if someone enrolled in our clinical trial went hiking and was hit by falling rocks, we probably would not blame the drug. Probably.
I am very happy to have restarted enrollment and look forward to seeing where this clinical trial adventure ends. Hopefully, these 6-7 years of my life (and lots of other people’s lives) will give the World a new drug to better treat cerebral malaria in children. Fingers crossed.